|
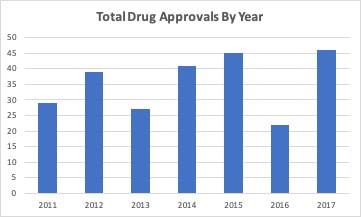
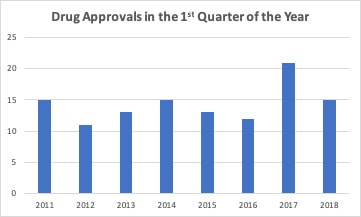
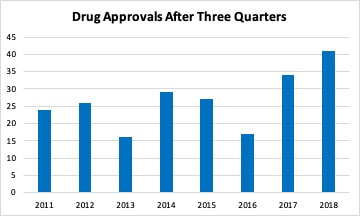
Last spring, I looked at the trend in the number of new drug approvals by the FDA. The FDA was coming off a year with a greatly increased number of approvals compared to the previous year. When looked at over time, it appeared this was a long term trend that built on streamlining and optimizing the drug approval process. Thus, 2017 was similar to 2015 and just a little higher than 2012 and 2014. So, compared to 2016, 2017 looked more in line with the 2014-2015 trend in drug approvals and 2016 had a reduced number compared to previous years. After one quarter, it appeared that 2018 was going to look more like 2016 with fewer drug approvals. I even wrote an editorial suggesting this but cautioned that it was still early to forecast the final volume. The FDA has been updating guidance that it issues for the development of drugs. As an example, new guidance for five neurological conditions was released at the beginning of the year. This new guidance along with new types of clinical trial methodologies such as adaptive trials have has effectively brought more drugs into the final stages of development. The FDA approved a greater number of drugs each month from May to September in 2018. The only exception was June, when they equaled the previous highest total. Through three quarters, the FDA is now on track to exceed the number of drugs approved in 2017 with the highest number of total approvals in seven years. There has also been an increase in the number of generic drugs approved. The FDA uses an October to September fiscal year and reported 781 drugs approved in fiscal year 2018 compared to 763 in fiscal 2017. As with branded drugs, the FDA has also been releasing guidance documents to streamline approval of generic drugsand to promote competition for single source drugs with no patent protection. The FDA’s generic approval program should increase the number of manufacturers and help stabilize drug supplies and lower prices.
Subscribe to the Pharmaceutical Pipeline Tracker for only $115 per month to take a deeper dive into the investigational drug universe: https://www.prescriberight.com/subscription-offers.html Search for budget busting drugs by clicking on our “Current list of drugs with PDUFA”date button to review upcoming approval deadlines and any drugs with priority designations. Follow this link to view our case studies: https://www.prescriberight.com/case-studies.html Contact us with specific requests or questions at https://www.prescriberight.com/contact.html Comments are closed.
|
Stay informed, subscribe to the
Prescribe Right Pharmaceutical Pipeline Tracker Latest Tweets from Prescribe Right
Archives
July 2023
|
Services |
Company |
Support |
© COPYRIGHT 2015. ALL RIGHTS RESERVED.
|


 RSS Feed
RSS Feed