|
RedHill Biopharma’s combination of amoxicillin, omeprazole, rifabutin (Brand Name: Talcia) for the eradication of H. pylori has a PDUFA Date of November 2, 2019. A Phase III trial demonstrated the combination of amoxicillin, omeprazole and rifabutin delivered an 84% eradication of H. pylori compared to 58% eradication with amoxicillin and omeprazole in dyspepsia patients with confirmed H. pylori infection. Of the thirty-six investigational drugs in the Pharmaceutical Pipeline Tracker, Talcia is the only drug targeting the eradication of H. pylori. It has FDA Priority Designations as a Fast Tracked Qualified Infectious Disease Product.
Short list for November. So, let’s take a quick look at the seven investigational drugs with December PDUFA Dates. Celgene/Acceleron’s luspatercept – PDUFA Date: Dec 4, 2019, a TGF-beta signaling modulator via subcutaneous injection targeting Beta thalassemia, Myelodysplastic Syndromes. Enzyvant’s RVT-802 – PDUFA Date: Dec 6, 2019, a tissue-based regenerative therapy via transplantation targeting pediatric congenital athymia. RVT-802 has four FDA Priority Designations: Fast Track, Breakthrough Therapy, Rare Pediatric Disease, and Regenerative Medicine Advance Therapy. Intra-Cellular Therapies’ lumateperone - PDUFA Date: Dec 27, 2019, an oral atypical antipsychotic to treat acute and residual schizophrenia. Lumateperon has an FDA Fast Track Priority Designation. Allergan’s ubrogepant – PDUFA Date: Dec 27, 2019, is an oral gepant to treat acute migraines. Eisai/Purdue’s lemborexant – PDUFA Date: Dec 27, 2019 is an oral orexin receptor antagonist targeting Insomnia. ViiV Healthcare/Janssen’s cabotegravir/rilpivirine – PDUFA Date: Dec 29, 2019, is an INSTI/NNRT delivered either orally or via Intramuscular injection as an HIV treatment. AMCP Nexus 2019 on Alzheimer’s Our October 24, 2019 post, Update on Investigational Drugs for Alzheimer ’s Disease, noted that progress was anticipated in the treatment of the disease this year. But the newswires have become largely radio silent. However, a comprehensive presentation on the diagnosis, treatment, and prevention of Alzheimer’s disease was recently given at AMCP Nexus 2019, according to a report filed by Jack Carafano from Day 1 of the conference. Here’s a link to Jack’s report. Regulatory Update
The FDA approved the combination of elexacaftor, ivacaftor, and tezacaftor (Trikafta, Vertex Pharmaceuticals) on October 21 for the treatment of patients age 12 years or older with cystic fibrosis (CF) and at least one F508del mutation in the CF transmembrane conductance regulator (CFTR) gene. The combination was approved almost 6 months early (3/19/2020 PDUFA date). Ascendis plans to file a BLA for TransCon hGH in the first half of 2020 and an MAA in in the second half of 2020 to treat pediatric growth hormone deficiency. TransCon hGH was designated an Orphan drug by the EMA. The EMA has granted teplizumab PRIME status for the prevention or delay of clinical type 1 diabetes Announced Research Updates Reata announced that in the 48-week, 103 patient Phase II, MOXIe trial, patients treated with omaveloxoloneimproved the modified Friedreich ataxia Rating Scale by 1.55 points compared to a worsening of 0.85 points with placebo in patients with Friedreich ataxia. In March Biogen announced that an interim analysis of the Phase III ENGAGE and EMERGE trials found a low likelihood that aducanumab would improve or slow cognition in Alzheimer’s patients, so Biogen discontinued both trials in March 2019. Biogen analyzed additional data from 3,285 patients and found an improvement in 547 patients enrolled in EMERGE that received the highest dose of the drug. Aducanumab reduced the decline in CDR-SB (23%), MMSE (15%), ADAS-Cog 13 (27%) and ADCS-ADL-MCI (40%). The expanded cohort of high dose patients in ENGAGE did not demonstrate an improvement compared to placebo. Novartis announced that in two 12-week, 700 patient Phase III trials (ZEAL 1 and ZEAL 2), treatment with fevipiprant did not improve FEV1 compared to placebo in patients with moderate asthma. Fevipiprant is being evaluated in preventing exacerbation in moderate to severe asthma in the Phase III LUSTER 1 and 2 trials. Protalix announced interim results from 16 patients in the 12-month, 22 patient, Phase III, open-label BRIDGE trial, showed a decline in eGFR from -5.10 mL/min/1.73m2 per year on stable doses of agalsidase alfa to -0.23 mL/min/1.73m2 per year on pegunigalsidase. Protalix plans to submit a BLA for pegunigalsidase in 1Q20. We heard much about drugs in development to treat or delay the onset of Alzheimer’s disease during 2018. There are twenty-five investigational drugs targeting Alzheimer's disease currently in the Pharmaceutical Pipeline Tracker. None of the drugs have PDUFA dates at this time although there are five drugs with FDA Priority Designations.
Progress was anticipated in the treatment of the disease this year. But the newswires have become largely radio silent. Following is an example of what we did hear. In March Biogen announced that an interim analysis by an independent committee of two Phase III trials involving patients with mild cognitive impairment due to Alzheimer's disease and mild Alzheimer's disease dementia. The interim futility analysis was based on data from 1,748 patients and found a low likelihood that aducanumab would improve or slow cognition in Alzheimer’s patients, so Biogen discontinued both trials in March 2019. In the meantime, Biogen analyzed addition data from 3,285 patients, of which 2,066 had completed the full 18 months of treatment and found a reduction in cognition reduction compared to placebo in the one of the trials’ data. The benefit was primarily from an analysis of an expanded cohort of patients which found a reduction in the decline in cognition with the higher dose of aducanumab as demonstrated by improvements over 78 weeks in 547 patients compared to placebo in CDR-SB (23%), MMSE (15%), ADAS-Cog 13 (27%) and ADCS-ADL-MCI (40%). The expanded cohort of high dose patients did not demonstrate an improvement compared to placebo. Biogen will offer patients enrolled in the two trials continued access to aducanumab, along with the long-term extension study for a Phase 1b study, and safety study of the data from one of the previous Trials. Biogen plans to file a BLA for aducanumab in early 2020. Ten trials of investigational drugs targeting Alzheimer’s have been discontinued. Of those attempts still in progress a number of pathways are under investigation. For example: monoclonal antibodies, BACE Inhibitors, amyloid beta protein inhibitors, caprilic triglycerides, and beta ameloid reduction. One of the companies involved in the research has gone out of business. Another has no ongoing US trials. Most of the ongoing Phase III trials are slated to deliver results no earlier than late 2020. October 2019 Regulatory Update
The FDA Antimicrobial Drugs Advisory Committee voted 14-2 to recommend approval of cefiderocol for the treatment of complicated urinary tract infections. The FDA accepted the NDA for rimegepant, for the treatment and prevention of migraines and set a PDUFA date in March 2020. The FDA accepted the NDA for triheptanoin, for the treatment of long-chain fatty acid oxidation disorders (LC-FAOD) and set a PDUFA target date of 7/31/2020. The FDA accepted the BLA for Trastuzumab deruxtecan, for the treatment HER2-positive metastatic breast cancer and set a PDUFA date in March 2020. The FDA awarded a Rare Pediatric Disease designation to sepofarsen for the treatment of Leber’s congenital amaurosis 10. CHMP advised the EMA not to approve quizartinib due to a lack of evidence to support a survival benefit. Announced Research Updates Principia announced that in a 24-week, 15 patient, Phase II, open-label trial, 60% of patients treated with PRN1008 achieved CDA and 40% achieved complete remission in patients with moderate to severe pemphigus. Lilly announced that in the 567 patient, Phase III SEQUOIA trial, the addition of pegilodecakin to FOLFOX (folinic acid, 5-FU, oxaliplatin) did not improve overall survival compared to FOLFOX alone in patients with metastatic pancreatic cancer whose disease had progressed despite a gemcitabine-containing regimen. UCB announced that in the 16-week, 570 patient, Phase III, BE VIVID trial, more patients treated with bimekizumab achieved a 90% improvement in the Psoriasis Area and Severity Index (PASI 90) and Investigator Global Assessment (IGA) score of clear or almost clear (IGA 0/1) than placebo or ustekinumab in patients with moderate-to-severe chronic plaque psoriasis. Congo is using both the J&J and Merck vaccines during the 2019 Ebola outbreak. Published Research Updates In two Phase III trials (SAKURA 1 and SAKURA 2) a total of 609 patients were treated with daxibotulinumtoxinA and achieved a reduction in their Glabellar line severity score at week 4 by at least two points in 73.6% vs 0% in SAKURA 1 and in 74% vs 1% in SAKURA 2 with line severity not returning to baseline until 27.7 weeks in SAKURA 1 and 26 weeks in SAKURA 2. In a 223 patient, Phase IIb trial, treatment with GC4419 compared to placebo reduced development of severe radiation-induced oral mucositis (SOM) (43% vs 65%) and duration of SOM (1.5 vs 19 days) in patients with locally advanced oral cavity or oropharynx cancer receiving seven weeks radiation therapy plus cisplatin. We have been focusing on Cancer with our last updates focused on Breast Cancer and Lung Cancer.
We take a moment now to focus on the number one cause of death in the US, Heart Disease. There are eleven investigational drugs for Heart failure in the Pharmaceutical Pipeline Tracker. Of the 23 investigational drugs with PDUFA dates, none are targeting Heart Failure. However, three investigational drugs to treat Heart failure have FDA Priority Designations. Vericel’s ixmyelocel-T is a fully closed cell-processing system that expands mesenchymal stromal cells and macrophages, which create anti-inflammatory and pro-angiogenic factors to repair damaged tissue and is administered via transendocardial injection. Ixmyelocel-T has orphan drug status for dilated cardiomyopathy and Fast Track status for peripheral ischemia. It has also received a Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA along with a Rare Pediatric Disease designation. There are links to two published studies for Ixmyelocel-T in the Pharmaceutical Pipeline Tracker. Mesoblast’s MPC-150-IM is a mesenchymal precursor cell therapy that is administered via Intravenous Infusion in Phase III trials. MPC-150-IM is competing with Biocardia's autologous mesenchymal stem cell product. Mesoblast has the ongoing 600 patient, Phase III, DREAM HF-1 trial evaluating whether MPC-150-IM can reduce nonfatal heart failure-related major adverse cardiac events in moderate heart failure patients. The FDA has given MPC-150-IM a Rare Pediatric Disease and Regenerative Medicine Advanced Therapy (RMAT) priority designations. There is one published study for MPC-150-IM in the Pharmaceutical Pipeline Tracker. Ironwood Pharmaceuticals’ praliciguat is an oral soluble guanylate cyclase (sGC) stimulator that improves nitric oxide (NO) signaling, which is being developed for heart failure with preserved ejection fraction (HFpEF) and diabetic nephropathy. The drug is in an on-going Phase II Trial and has a Fast Track priority designation from the FDA. Subscribe to the Pharmaceutical Pipeline Tracker to review investigational drugs focused on Heart Failure. Read about safety, efficacy and the latest news. Take a look at published research links. Regulatory Update
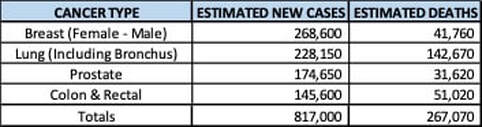
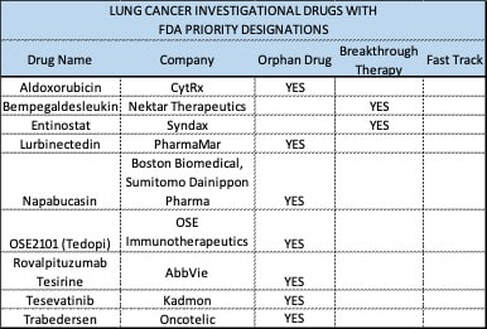
The FDA approved lasmiditan (Reyvow, Lilly) on 10/11/2019 for the treatment of acute migraines, with or without aura, in adult patients. Due to the occurrence of dizziness and sedation from CNS depression, patients are warned of potential impairment while taking lasmiditan and advised not to drive, operate machinery or take with alcohol or other CNS depressants for at least eight hours after taking the drug, even if they feel well enough to do so. The FDA announced that during clinical trials the most common ADR with lasmiditan were dizziness, fatigue, paresthesia and sedation. The FDA approved brolucizumab (Beovu, Novartis) on 10/8/2019 for the treatment of wet age-related macular degeneration (AMD). Brolucizumab is administered by intravitreal Injection every three months. WAC was set at $1,850 per dose, the same as aflibercept (Eylea), which is given more frequently. After the loading dose, analysts estimate the annual brolucizumab WAC at $16,000 for both eyes compared to $24,000 for aflibercept and and $40,000 for ranibizumab (Lucentis). The FDA approved afamelanotide (Scenesse, Clinuvel) on 10/8/2019 to treat phototoxic reactions in patients with erythropoietic protoporphyria. The FDA accepted the BLA for Viaskin Peanut for the treatment of peanut-allergic children in October 2019 and set a PDUFA date of 8/5/2020. Announced Research Updates NGM announced that in a 24-week, 38 patient, Phase II trial, treatment with aldafermin resulted in a 7.9% decrease in liver fat content compared to 2% decrease with placebo in patients with biopsy-confirmed NASH with F2-F3 liver fibrosis. Patients acted as their own controls in the REVERSE and RESCUE trials with one eye injected with lenadogen nolparvovec and the other eye receiving a sham injection. Both trials were done in patients with Leber Hereditary Optic Neuropathy (LHON) from the G11778A mutation in the mitochondrial ND4 gene. GenSight announced that in the 96-week, 37 patient, Phase III, REVERSE trial, lenadogen nolparvovec improved visual acuity from vision nadir by 15.4 letters in the treated eye, which was a non-significant difference compared to a 12.9 letter improvement in the eye that received a sham injection. GenSight announced that in the 96-week, 39 patient, Phase III, RESCUE trial, the eyes treated with lenadogen nolparvovec had an improvement in visual acuity of 24.9 letters in the ETDRS scale from vision nadir compared to a gain of 22.3 letters in the eyes that received sham injection. A study in monkeys determined that intravitreal injections of lenadogen nolparvovec into one eye will transfer the gene therapy to the other eye. This may explain the improvement of visual acuity in REVERSE and RESCUE, which would contradict the natural course of the disease in the untreated eye, where an improvement of 10 letters from Nadir is usually seen. Helixmith announced that in a 12-month extension of the 500 patient, Phase III trial involving 101 patients, VM202 decreased the average pain score by 0.9 points compared to placebo in patients with painful diabetic peripheral neuropathy. Redhill announced that in the 52-week, 30 patient, Phase III, open-label extension, MAP US2 trial, treatment with RHB-104 demonstrated a remission rate of 22.2% at week 52. Published Research Updates In the 2-year, 549 patient, Phase II FORWARD trial, patients treated with sprifermin 100 mcg every six months had an increase in femorotibial cartilage thickness of 0.05 mm and an increase of 0.04 mm when given every 12 months compared to placebo in patients with osteoarthritis. There was no improvement with either dosing regimen with 30 mcg of sprifermin compared to placebo. There were no improvements in WOMAC scores with any dose or frequency of sprifermin. In a 12-week, 65 patient, Phase III trial, 57.6% of patients treated with relugolix had a maximum Numerical Rating Scale (NRS) score of 1 or less compared to 3.1% with placebo in patients with moderate-to-severe uterine fibroid-associated pain. In terms of the impact on healthcare systems, the top four cancers, by estimated new cases, represent 57% of new cases within the top thirteen and 61% of the estimated deaths for this group. Our Thursday, September 12, 2019, update focused on Breast Cancer Today we focus on Lung Cancer. Lung Cancer, with the second most frequent occurrence of new cases, is also the deadliest with a 63% mortality rate. Prescribe Right’s Pharmaceutical Pipeline Tracker shows twenty-one investigational drugs focused on Lung Cancer. Seventeen are in Phase III trials. Several of these efforts are focused on multiple cancers. Ten are focused solely on non-small cell lung cancer, six of which are in Phase III trials. There are no investigational drugs focused on Lung Cancer within the twenty-four that have PDUFA Dates between November 2, 2019 and August 5, 2020. There are, however, nine drugs to treat Lung Cancer with Priority Designations from the FDA. Seven drugs have Orphan Drug designations and two of the nine are Breakthrough Therapies. Subscribe to the Pharmaceutical Pipeline Tracker to review investigational drugs focused on Lung Cancer. Read about safety, efficacy and the latest news. Take a look at published research links.
Regulatory Update
The FDA approved trifarotene cream (Aklief, Galderma) on October 4 for the treatment of acne vulgaris. The FDA has granted Fast Track status to imetelstat for the treatment of adult patients with relapsed or refractory myelofibrosis, including whose disease has relapsed after or is refractory to janus kinase inhibitor treatment. Announced Research Updates MacroGenics announced that in a 92 patient, Phase II trial, treatment with margetuximab plus pembrolizumab resulted in an overall response rate of 21.7% and progression-free survival of 2.7 months in patients with metastatic HER2-positive gastroesophageal adenocarcinoma previously treated with trastuzumab and chemotherapy. BeiGene announced interim data from a 104 patient, Phase II, open label trial, where treatment with tislelizumab resulted in an overall response rate of 23.1% in Chinese and South Korean patients with with PD-L1+ locally advanced or metastatic urothelial carcinoma previously treated with a platinum-containing chemotherapy regimen. Resverlogix announced that in the Phase III, BETonMACE trial, apabetalone added to standard of care did not reduce major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or stroke) compared to placebo in high-risk patients with type 2 diabetes, recent acute coronary syndrome, and low HDL cholesterol. Amgen announced interim data from 12 of 76 patients enrolled in a Phase I, open-label, dose-ranging trial, received the highest dose of AMG 51 (960 mg) with 1 achieving a partial response and 10 achieving stable disease in previously treated patients with KRAS G12C-mutated colorectal cancer. Elevar announced that in the 460 patient, Phase III ANGEL study, treatment with rivoceranib plus best supportive care did not improve overall survival compared to plus best supportive care only (5.8 vs 5.1 months) in the overall population of patients with gastric or gastroesophageal junction cancer who have failed at least 2 prior lines of therapy. Overall survival was improved in 185 patients that had received 3 or more courses of chemotherapy before treatment with rivoceranib compared to placebo (6.43 vs 4.73 months). Shionogi announced that in the 14-day, 452 patient, Phase III, APEKS-NP trial, the mortality rate with cefiderocol was 12.4% compared to 11.6% with meropenem, which met non-inferiority criteria, in patients with nosocomial pneumonia. Rebiotix announced results from a 24-month, 95 patient, Phase II open-label, extension of the PUNCH CD trial, where 91% of patients that did not develop a CDI at 8 weeks remained CDI-free at 24 months post-RBX2660 treatment. Published Research Updates In a 160 patient, Phase I/Ib, open-label trial, umbralisib plus ublituximab resulted in an overall response rate of 46% with 17% achieving a complete response in patients with chronic lymphocytic lymphoma or non-Hodgkin lymphoma. In the 48-week, 103 patient, Phase IIb PRAISE trial, patients treated with pamrevlumab had a reduction of 2.9% in the percentage of predicted FVC compared to 7.2% decrease with placebo in patients with mild-to-moderate idiopathic pulmonary fibrosis. Disease progression was seen in 10% of patients treated with pamrevlumab compared to 31.4% of patients in the placebo group. In a 12-week, 267 patient, Phase IIb trial, 43.8% of patients treated with abrocitinib 200 mg and 29.6% that received 100 mg achieved an Investigator Global Assessment (IGA) score of clear or almost clear skin and had a 2 point or > improvement in their IGA score compared to 5.8% with placebo in patients with moderate to severe atopic dermatitis. In a 12-week, 382 patient, Phase IIb trial, patients treated with ligelizumab achieved a weekly hives-severity score of 0 in 30% of patients treated with 24 mg, 51% treated with 72 mg and 42% treated with 240 mg compared to 26% treated with omalizumab and none with placebo in patients with chronic spontaneous urticaria. In a 30-day, 828 patient, Phase III trial, treatment with selepressin did not improve ventilator- and vasopressor-free days compared to placebo in patients with septic shock receiving norepinephrine. In a 6-month, 94 patient, Phase III, open-label, SONICS trial, 82% of patients treated with levoketoconazole had their mean 24-h urinary free cortisol (mUFC) normalized during a titration phase in patients with Cushing’s syndrome. During the 6-month maintenance phase of the trial 31% of patients achieved and maintained normal mUFC. Approvals
Announced Research Updates
Helixmith announced that in a 3-month, 500 patient, Phase III trial, VM202 did not improve the verage pain score compared to placebo in patients with painful diabetic peripheral neuropathy. GenSight announced that in the 96-week, 36 patient, Phase III, RESCUE trial, the eyes treated with lenadogen nolparvovec had an improvement in visual acuity of 24.9 letters in the ETDRS scale compared to a gain of 22.3 letters in the eyes that received sham injection in patients with LHON who had suffered vision loss of less than 6 months. Patients acted as their own controls with one eye injected with lenadogen nolparvovec and the other eye receiving a sham injection. BeiGene announced interim data from a 54 patient, Phase II, open label, Chinese trial, where treatment with tislelizumab resulted in an overall response rate of 66.7% in patients with advanced lung cancer. Urovant announced that in a 40-week blinded extension of the EMPWUR trial that followed 506 patients from the original trial population, treatment with vibegron continued to reduce micturitions per 24 hours and urge urinary incontinence episodes per 24 hours. Pfizer announced that in a 12-week, 391 patient, Phase III, JADE MONO-2 trial, more patients treated with abrocitinib 100 mg and 200 mg achieved an Investigator Global Assessment (IGA) score of clear or almost clear skin, had a 2 point or > improvement in the IGA score and had more patients achieve at least a 75% or greater improvement in their Eczema Area and Severity Index (EASI) score compared to placebo in patients with moderate to severe atopic dermatitis. Daiichi Sankyo and AstraZeneca announced that interim results from 23 patients from the Phase II, open-label, MOUNTAINEER trial, treatment with tucatinib in combination with trastuzumab resulted in an objective response rate of 52.2% in patients with HER2-positive (HER2+), RAS wild-type metastatic colorectal cancer (mCRC) after treatment with first- and second-line standard-of-care therapies. Astellas and Seattle Genetics announced interim results from 45 patients in a Phase I, open label trial, where treatment with enfortumab vedotin plus pembrolizumab resulted in a 71% objective response rate in patients with locally advanced or metastatic urothelial cancer who progressed after receiving platinum-containing chemotherapy and a PD-1 or PD-L1 inhibitor. Avanir announced that in a 12-week, 522 patient, Phase III trial, treatment with deudextromethorphan/quinidine did not improve the Cohen-Mansfield Agitation Inventory score compared to placebo in patients with agitation associated with dementia of Alzheimer's disease. Lilly announced interim data from the 55 patients in the Phase I/II LIBRETTO-001, open-label trial with RET-mutant medullary thyroid cancer patients who had previously received cabozantinib and/or vandetanib, where treatment with selpercatinib resulted in a 56% objective response rate. Immunomedics announced interim data from 35 patients in the Phase II, open label, TROPHY-U-O1 trial found that treatment with sacituzumab govitecan resulted in an ORR of 29% in patients with metastatic urothelial carcinoma who progressed after treatment with platinum-based therapy and checkpoint inhibitors. ImmunoGen announced that in the 366 patient, Phase III, FORWARD I trial, mirvetuximab soravtansine did not improve progression-free survival compared to standard chemotherapy in patients with folate receptor alpha-positive, platinum-resistant ovarian cancer and had received up to three prior lines of therapy. Published Research Updates In the 12-month, 501 patient, dose ranging, Phase II, ORION-1 trial, the reduction in LDL with inclisiran was 29.5% to 38.7% with a single dose of the drug and 29.9% to 46.4% with a second dose at 3-months in patients with elevated LDL-C despite maximally tolerated statin therapy. In a 52-week, 368 patient, Phase III, open-label trial, treatment with esaxerenone decreased systolic blood pressure by 23.1 mm Hg, diastolic blood pressure by 12.5 mm Hg and 66% of patients were maintained on monotherapy in Japanese patients with essential hypertension. In a 1,140 patient, Phase III trial, veliparib added to carboplatin and paclitaxel followed by veliparib maintenance therapy improved progression-free survival compared to carboplatin plus paclitaxel induction therapy alone (34.7 months vs 22.0 months) in patients with previously untreated stage III or IV high-grade serous ovarian carcinoma. |
Stay informed, subscribe to the
Prescribe Right Pharmaceutical Pipeline Tracker Latest Tweets from Prescribe Right
Archives
July 2023
|
Services |
Company |
Support |
© COPYRIGHT 2015. ALL RIGHTS RESERVED.
|


 RSS Feed
RSS Feed