Brief Description– Determining the treatment options for various clinical indications can be cumbersome. In the example of Heart Failure, there are nine pharma companies involved in developing 10 investigational entities. Ten different drug classes are involved in these efforts. Rather than seeking information separately from multiple sources (FDA, pharma companies, primary literature) the Pharmaceutical Pipeline Tracker aggregates all the information in a single content source.
Actors– Hospital pharmacists with P&T committee investigational drug update responsibilities.
Preconditions – A list of the indications which require the most resource commitments from the organization. Access to a web-based subscription to the Pharmaceutical Pipeline Tracker
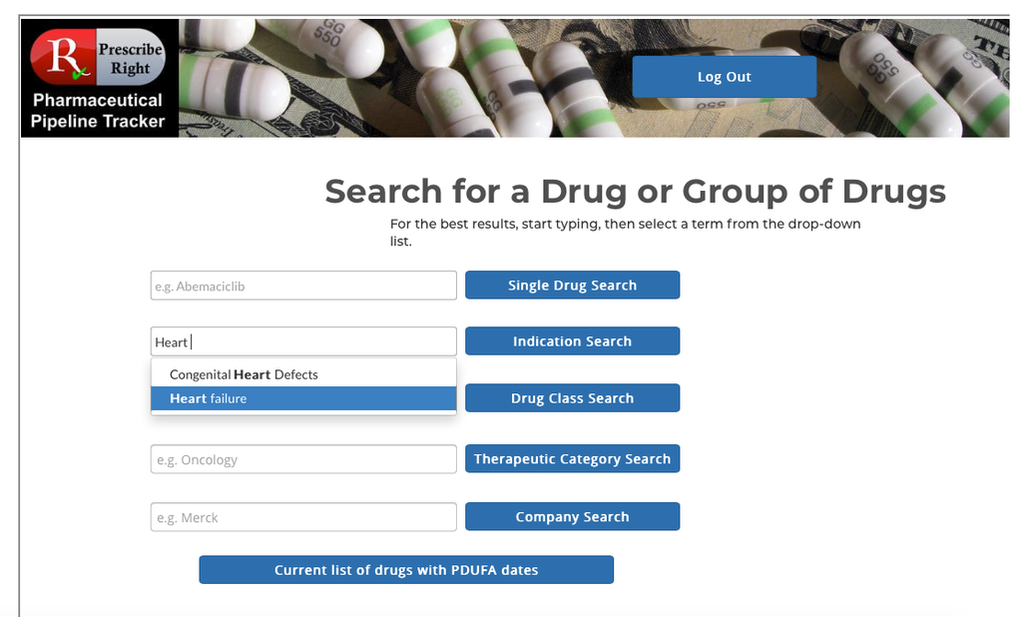
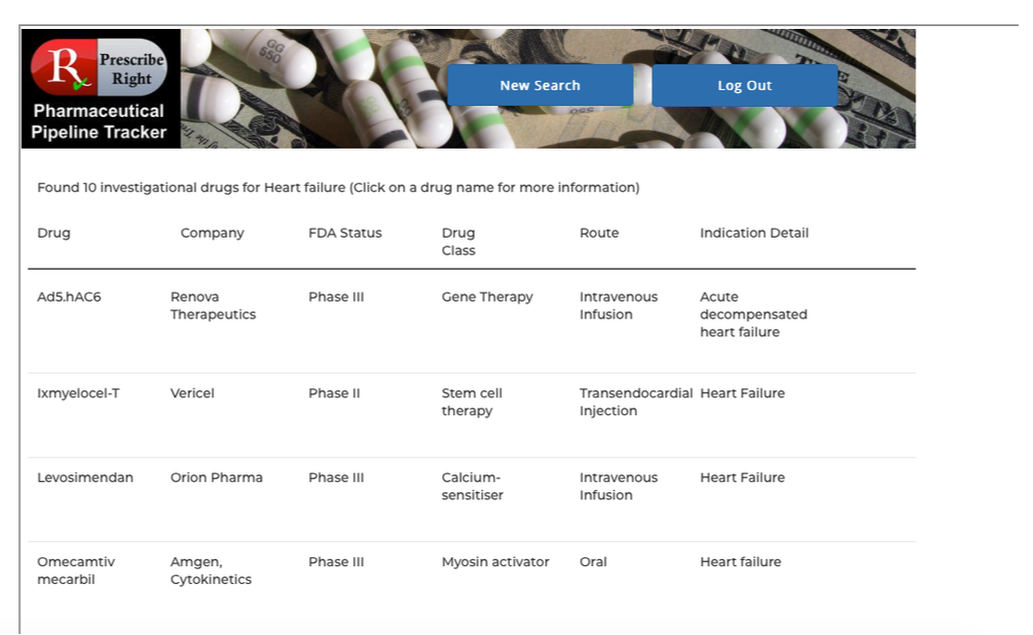
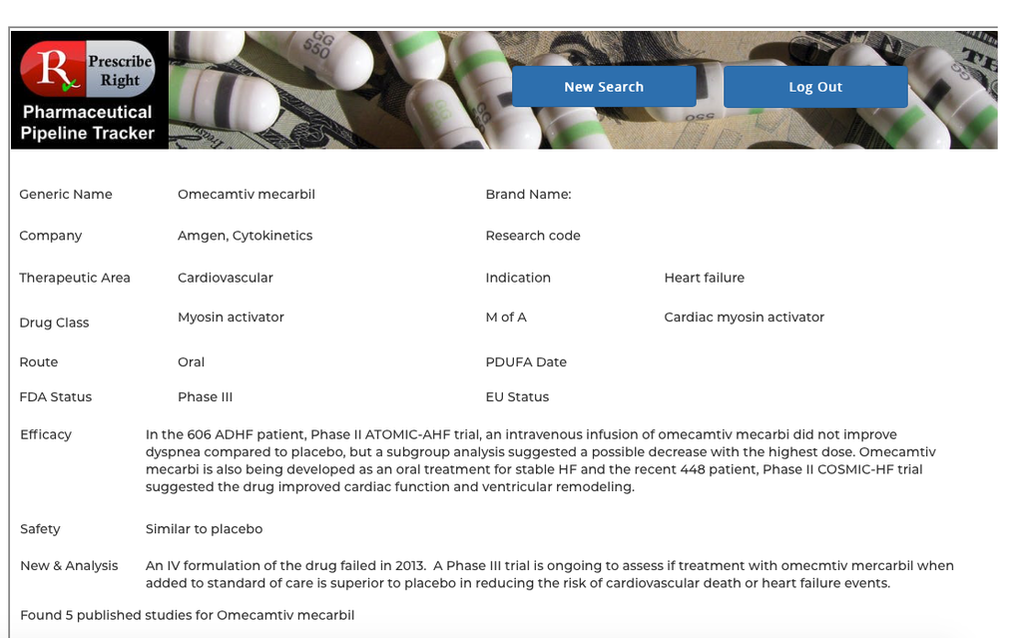
Basic Flow– At any computer, laptop, or mobile device open a web browser and enter www.prescriberight.com in the search field then hit the enter key on the keyboard. The Home page opens. On the command line hover over “Pharmaceutical Pipeline Tracker” and click on “Access the Tracker” on the drop-down menu. Then begin entering the indication in the search box next to the Indication Search button. A scrollable word wheel appears from which to choose the indication for which you wish to see a list of drugs in development. Clicking on any drug name provides access to that drug’s monograph containing the following data elements: generic name, brand name (if available), pharma company, research code, indication, drug class, route, PDUFA Date (if available), FDA and EU status. Statements on efficacy and safety are present. Published research studies (if available) are accessible via URL links to PubMed.
Actors– Hospital pharmacists with P&T committee investigational drug update responsibilities.
Preconditions – A list of the indications which require the most resource commitments from the organization. Access to a web-based subscription to the Pharmaceutical Pipeline Tracker
Basic Flow– At any computer, laptop, or mobile device open a web browser and enter www.prescriberight.com in the search field then hit the enter key on the keyboard. The Home page opens. On the command line hover over “Pharmaceutical Pipeline Tracker” and click on “Access the Tracker” on the drop-down menu. Then begin entering the indication in the search box next to the Indication Search button. A scrollable word wheel appears from which to choose the indication for which you wish to see a list of drugs in development. Clicking on any drug name provides access to that drug’s monograph containing the following data elements: generic name, brand name (if available), pharma company, research code, indication, drug class, route, PDUFA Date (if available), FDA and EU status. Statements on efficacy and safety are present. Published research studies (if available) are accessible via URL links to PubMed.
Alternate Flows– In addition to the Indication Search capabilities as described above, a search button is present for a single investigational drug name search. In the Single Drug Search box, begin typing a drug name, click on the drug name from the word wheel when it appears and click the Single Drug Search button. The drug’s individual monograph is displayed containing the following data elements: generic name, brand name (if available), pharma company, research code, indication, drug class, route, PDUFA Date (if available), FDA and EU status. Statements on efficacy and safety are present. Published research studies (if available) are accessible via URL links to PubMed.
Drug Class, Therapeutic Category (i.e. oncology), and pharma company searches result in a listing of all investigational drugs within that category. Clicking on any drug name takes the user to that drug’s individual monograph containing the following data elements: generic name, brand name (if available), pharma company, research code, indication, drug class, route, PDUFA Date (if available), FDA and EU status. Statements on efficacy and safety are present. Published research studies (if available) are accessible via URL links to PubMed. Click on the back arrow returns to the list of drugs for continued study of the category.
Exception Flows– User Name and Passwords must be spelled correctly. Begin searches by typing the first letters of the search word in the indication search box, then choose from the word wheel list and click on the appropriate indication, then click on the blue Indication Search button.
Post Conditions– Users will have sufficient background information to present recommendations to the P&T Committee and/or any department seeking drug utilization recommendations. Specifically, the question of whether or not to include a new or about to be released investigational drug in the formulary. Advisory information about additional staff training, laboratory testing, monitoring, and requisite patient education should be noted.
Services |
Company |
Support |
© COPYRIGHT 2015. ALL RIGHTS RESERVED.
|