Brief Description– The Prescription Drug User Fee Act (PDUFA) was created by Congress in 1992 and authorizes FDA to collect fees from companies that produce certain human drug and biological products. Since the passage of PDUFA, user fees have played an important role in expediting the drug approval process.
PDUFA must be re-authorized every five years, and was renewed in 1997 (PDUFA II), 2002 (PDUFA III), 2007 (PDUFA IV), and 2012 (PDUFA V) and 2017 (PDUFA VI). On August 18, 2017, the President signed into law the Food and Drug Administration Reauthorization Act (FDARA), which includes the reauthorization of PDUFA through September 2022. PDUFA VI will provide for the continued timely review of new drug and biologic license applications.
Under PDUFA, the FDA is usually given 10 months to review a new drug application. However, if a drug is designated for priority review, the FDA is given six months to review that drug.
Only a select number of drugs are deemed eligible for priority review. Typically, those given priority represent significant medical breakthroughs or offer treatment where few or no other options exist. Priority review is often granted to drugs that aim to treat serious medical conditions, though drugs that treat less serious conditions are also eligible for priority review if they meet the criteria. Review deadlines begin on the date that a new drug application is accepted by the FDA. In most cases drugs with a Priority Review are high cost specialty drugs that require special storage, administration technique, monitoring or acquisition barriers that require planning and coordination.
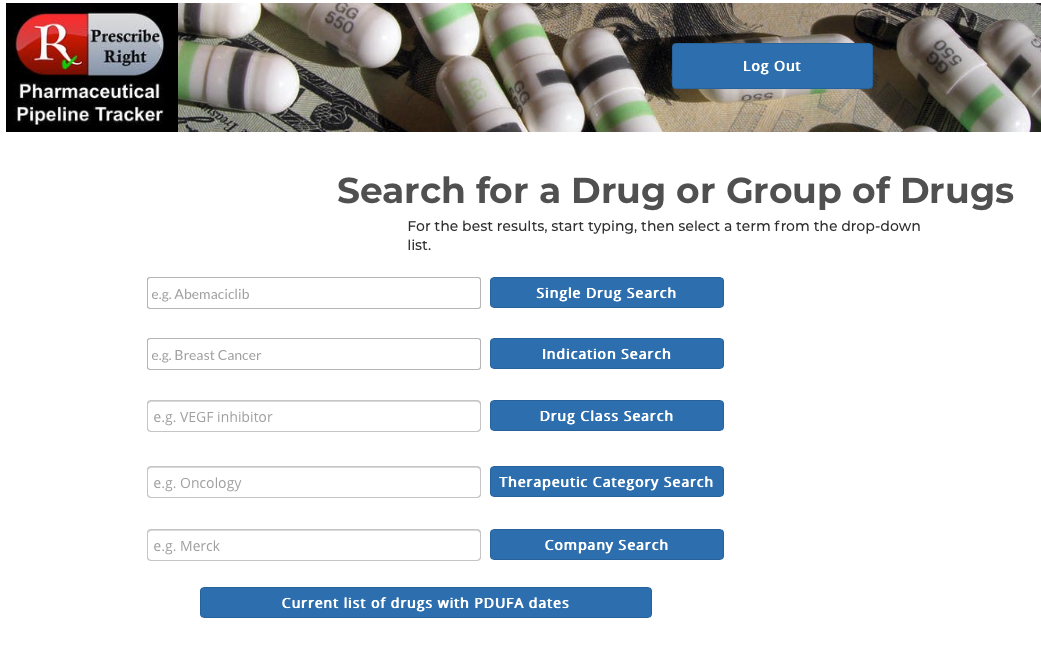
Rather than seeking a listing of investigational drugs with PDUFA Dates separately from multiple sources (FDA, pharmacy wholesalers, pharma companies, stock analysts, primary literature) the Pharmaceutical Pipeline Tracker provides that information at the click on a single button from our main search page. Access to any Priority Designations are listed.
Actors – Hospital pharmacists that provide recommendations for patient treatment, are responsible for providing investigational drug updates and reviews to the P&T Committee or have drug budget responsibilities.
Preconditions –Access to a web-based subscription to the Pharmaceutical Pipeline Tracker
Basic Flow – At any computer, laptop, or mobile device open a web browser and enter www.prescriberight.com in the search field then hit the enter key on the keyboard. The Home page opens. On the command line hover over “Pharmaceutical Pipeline Tracker” and click on “Access the Tracker” on the drop-down menu. Type in your assigned user name and password. Then hit the Login button. The main search screen appears at the bottom of which is the “Current List of Drugs with PDUFA Dates” button.
PDUFA must be re-authorized every five years, and was renewed in 1997 (PDUFA II), 2002 (PDUFA III), 2007 (PDUFA IV), and 2012 (PDUFA V) and 2017 (PDUFA VI). On August 18, 2017, the President signed into law the Food and Drug Administration Reauthorization Act (FDARA), which includes the reauthorization of PDUFA through September 2022. PDUFA VI will provide for the continued timely review of new drug and biologic license applications.
Under PDUFA, the FDA is usually given 10 months to review a new drug application. However, if a drug is designated for priority review, the FDA is given six months to review that drug.
Only a select number of drugs are deemed eligible for priority review. Typically, those given priority represent significant medical breakthroughs or offer treatment where few or no other options exist. Priority review is often granted to drugs that aim to treat serious medical conditions, though drugs that treat less serious conditions are also eligible for priority review if they meet the criteria. Review deadlines begin on the date that a new drug application is accepted by the FDA. In most cases drugs with a Priority Review are high cost specialty drugs that require special storage, administration technique, monitoring or acquisition barriers that require planning and coordination.
Rather than seeking a listing of investigational drugs with PDUFA Dates separately from multiple sources (FDA, pharmacy wholesalers, pharma companies, stock analysts, primary literature) the Pharmaceutical Pipeline Tracker provides that information at the click on a single button from our main search page. Access to any Priority Designations are listed.
Actors – Hospital pharmacists that provide recommendations for patient treatment, are responsible for providing investigational drug updates and reviews to the P&T Committee or have drug budget responsibilities.
Preconditions –Access to a web-based subscription to the Pharmaceutical Pipeline Tracker
Basic Flow – At any computer, laptop, or mobile device open a web browser and enter www.prescriberight.com in the search field then hit the enter key on the keyboard. The Home page opens. On the command line hover over “Pharmaceutical Pipeline Tracker” and click on “Access the Tracker” on the drop-down menu. Type in your assigned user name and password. Then hit the Login button. The main search screen appears at the bottom of which is the “Current List of Drugs with PDUFA Dates” button.
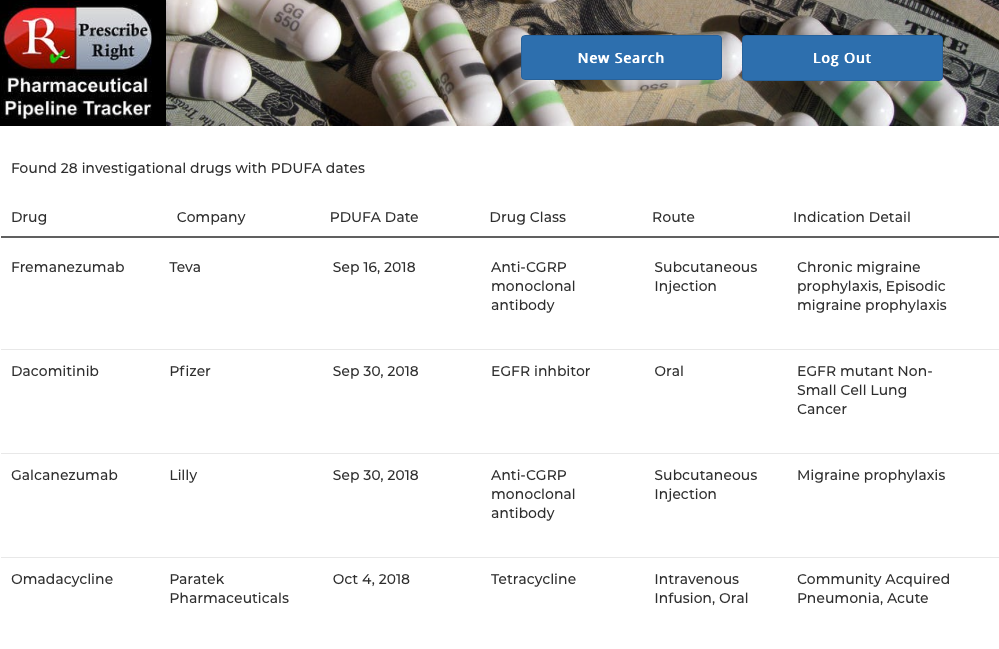
Click on that button and the following screen appears containing the scrollable list of drugs with PDUFA dates:
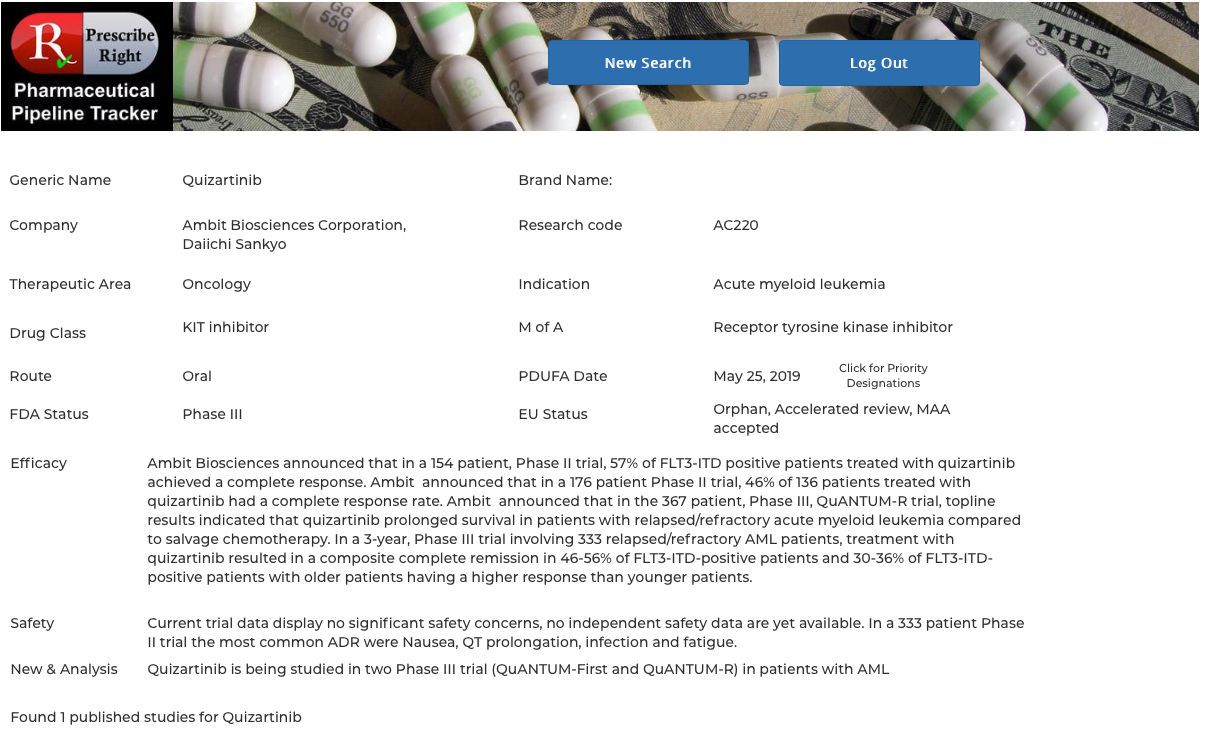
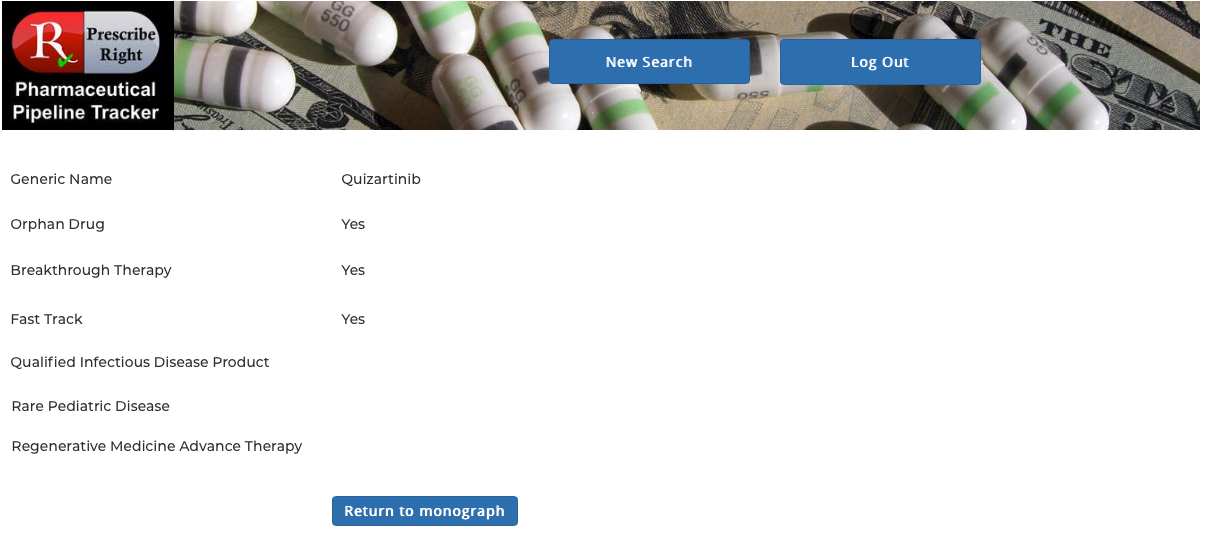
Clicking on any drug name provides access to that drug’s monograph containing the following data elements: generic name, brand name (if available), pharma company, research code, indication, drug class, route, PDUFA Date (if available) with Priority Designations, FDA and EU status. Statements on efficacy and safety are present. Published research studies (if available) are accessible via URL links to PubMed. Priority Designations, if any, are available by clicking on “Click for Priority Designations” next to the PDUFA Date. To return to the list click on the "Return to Monograph" button. View as many drugs as you wish as many times as you wish.
Alternate Flows – In addition to the list of drugs with PDUFA Dates as described above, search buttons are present for Drug Class, Therapeutic Category (i.e. oncology), and pharma company searches resulting in a listing of all investigational drugs within that search category. Clicking on any drug name takes the user to that drug’s individual monograph containing the following data elements: generic name, brand name (if available), pharma company, research code, indication, drug class, route, PDUFA Date (if available), FDA and EU status. Statements on efficacy and safety are present. Published research studies (if available) are accessible via URL links to PubMed. Click on the back arrow returns to the list of drugs for continued study of the category.
Exception Flows – User Name and Passwords must be spelled correctly. Begin searches by typing the first letters of the search word in the indication search box, then choose from the word wheel list and click on the appropriate indication, then click on the blue Indication Search button.
Post Conditions – Users will have background information to present recommendations to the P&T Committee and/or any department seeking drug treatment recommendations. Specifically, the question of whether or not to include a new or about to be released investigational drug with a potential high cost in the formulary. The information can also be used to educate and discuss an investigational drug with a patient or physician that is seeking a drug under a “Compassionate Use” or “Right to Try” protocol. Advisory information about additional staff training, laboratory testing, monitoring, and requisite patient education should be noted.
Exception Flows – User Name and Passwords must be spelled correctly. Begin searches by typing the first letters of the search word in the indication search box, then choose from the word wheel list and click on the appropriate indication, then click on the blue Indication Search button.
Post Conditions – Users will have background information to present recommendations to the P&T Committee and/or any department seeking drug treatment recommendations. Specifically, the question of whether or not to include a new or about to be released investigational drug with a potential high cost in the formulary. The information can also be used to educate and discuss an investigational drug with a patient or physician that is seeking a drug under a “Compassionate Use” or “Right to Try” protocol. Advisory information about additional staff training, laboratory testing, monitoring, and requisite patient education should be noted.
Services |
Company |
Support |
© COPYRIGHT 2015. ALL RIGHTS RESERVED.
|