|
There are two drugs with September PDUFA dates: tenapanor, one of nine investigational Nephrology drugs and semaglutide, one of twenty-six investigational Endocrinology drugs.
Ardelyx’ tenapanor’s PDUFA Date is 9/12/2019, and is targeting hyperphosphatemia in dialysis patients, IBS with constipation. This NHE3 Inhibitor blocks the NHE3 transporter in the GI tract to reduce the absorption of dietary sodium, which reduces phosphate absorption and increases the amount of fluid in the gut. Tenapanor has the potential to reduce pill burden in reducing serum phosphorus, but may have a higher incidence of diarrhea. Research published in the March 7, 2019, Journal of the American Society of Nephrology concluded “Tenapanor significantly reduced elevated serum phosphate in patients with hyperphosphatemia receiving maintenance hemodialysis. Adverse effects were limited to those induced by its known mechanism of action, which increases stool sodium and water content.” Novo Nordisk’s oral semaglutide has a September 20, 2019, PDUFA date. The drug is indicated for Type 2 Diabetes as a GLP-1 receptor agonist. The FDA approved the weekly subcutaneous injection on 12/5/17 for the treatment of Type 2 Diabetes. If approved, it would be the first oral GLP-1 receptor agonist and a potential game-changer for patients who wish to avoid an injection. Novo has announced the results from ten Phase III trials demonstrating a reduction in HbA1c and weight. Results of seven of the trials have been published. In June we provided a brief summary of the 10 trials. In the Pharmaceutical Pipeline Tracker, you will find additional information about trials and links to the seven published studies. The drug has been compared to placebo, empagliflozin, sitagliptin, liraglutide and dulaglutide with favorable results in the Phase III trials. A Phase II trial demonstrated similar efficacy with a daily oral tablet as achieved with a weekly injection of semaglutide. The adverse effect profile is similar to injectable GLP-1 receptor antagonists with nausea and vomiting being the most frequent ADR. There does not appear to be another oral drug in this class in the late stages of development. Lilly’s tirzepatide, an injectable GIP and GLP-1 receptor agonist is the most advanced drug behind oral semaglutide. Regulatory Update
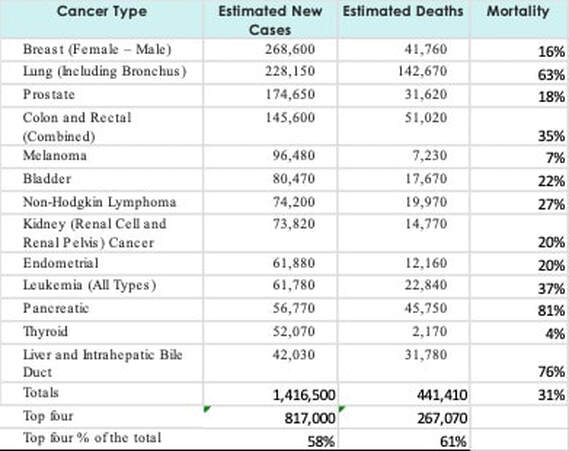
The FDA approved both oral and intravenous formulations of lefamulin (Xenleta, Nabriva Therapeutics) on 8/19/2019 for the treatment of community-acquired pneumonia. The WAC price per day for the IV dose is $205 and the oral dose is $275 per day. The FDA rejected Sarepta Therapeutics' golodirsen (Vyondys 53) for the treatment of Duchenne muscular dystrophy amenable to exon 53 skipping due to the risk of infection with intravenous infusion ports and renal toxicity seen in pre-clinical studies. Sarepta has shown that golodirsen will increase dystrophin levels, but has not completed studies to demonstrate animprovement in muscle function. The FDA accepted the NDA for VX-445 (elexacaftor, tezacaftor and ivacaftor) for the treatment of cystic fibrosis in patients with one F508del mutation and one minimal function mutation or two F508del mutations. A PDUFA date has been set for 3/19/2020. The FDA granted Fast Track status to Moderna’s investigational Zika vaccine, mRNA-1893. Celgene plans to submit a BLA for lisocabtagene maraleucelfor the treatment of multiple myeloma in the first half of 2020. DBV submitted a BLA for Viaskin Peanut for the treatment of peanut-allergic children. The FDA accepted the BLA for zanubrutinib for the treatment of mantle cell lymphoma and assigned a PDUFA date of 2/27/2020. Celgene submitted a BLA for Zbb2121 for the treatment of multiple myeloma that has reappeared after remission or is resistant to treatment. Announced Research Updates ViiV announced that in the 48-week, 1,045 patient, Phase III ATLAS-2M trial, cabotegravir and rilpivirine given eight weeks was non-inferior to monthly injections in maintaining HIV-1 RNA < 50 copies/mL. Published Research Updates In the 465 patient, Phase II/III ZEUS trial, 64.7% of patients treated with injectable fosfomycin achieved clinical cure and microbiologic eradication that was non-inferior to the 54.5% overall success achieved with piperacillin-tazobactam in patients with urinary tract infection (cUTI) or acute pyelonephritis (AP). The original ZEUS data was calculated based on the 2015 FDA Guidance for the development of antibiotics to treat cUTI/AP. Nabriva announced that in a reassessment of the ZEUS data based on the 2018 FDA Guidance, which reduced the microbial eradication threshold, 78.6% for patients in the fosfomycin group achieved overall success compared to 33.3% of patients in the piperacillin-tazobactam group, in patients with piperacillin-tazobactam-resistant infections. In patients with piperacillin-tazobactam-susceptible pathogens, the overall success rate was 64.7% for patients in the fosfomycin group and 56.7% for patients in the piperacillin-tazobactam group. In the 6,545 patient, Phase III trial, Norvartis’ serelaxinwas no different than placebo in preventing further decompensation over the first 5 days (6.9% vs 7.7%) or in reducing CV death at 6-months (8.7% vs 8.9%). In a 38 patient, Phase II trial, treatment with Peregrine Pharmaceuticals’bavituximab plus sorafenib resulted in a median time to progression of 6.7 months in patients with advanced hepatocellular carcinoma. In a 30-patient, Phase Ib, open-label, trial, treatment with Roche’s taselisib plus tamoxifen resulted in progression free survival of 3.7 months in patients with ER-positive, metastatic breast cancer who had failed prior endocrine therapy. According to NIH’s National Cancer Institute, the following chart shows the top thirteen cancer types in the US as of February 2019 sorted by estimated new cases. In terms of impact on healthcare systems, the top four cancers by estimated new cases represent 57% of new cases within the top thirteen and 61% of the estimated deaths for this group.
Lung Cancer, with the second most frequent occurrence of new cases, is also the deadliest with a 63% mortality rate. Prescribe Right’s Pharmaceutical Pipeline Tracker shows twenty-one investigational drugs focused on Lung Cancer. Seventeen are in Phase III trials. Several of these efforts are focused on multiple cancers. Ten are focused solely on non-small cell lung cancer, six of which are in Phase III trials. There are no investigational drugs focused on Lung Cancer within the twenty that have PDUFA Dates between August 27, 2019 and May 14, 2020. Breast cancer has the most occurrences of new cases and the third lowest mortality rate (16%). Prescribe Right’s Pharmaceutical Pipeline Tracker shows 18 investigational drugs focused on Breast Cancer. Fourteen of the drugs are in Phase III trials. Several of these efforts are focused on multiple cancers including non-small cell lung cancer. The oncology therapeutic category is the largest therapeutic category within the Pharmaceutical Pipeline Tracker with 133 investigational Oncology drugs. Regulatory Update
The FDA approved pretomanid (TB Alliance) on 8/14/2010 in conjunction with bedaquiline and linezolid for the treatment of adults with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant tuberculosis. The FDA approved pitolisant (Wakix, Harmony Biosciences) on 8/15/2019 for the treatment of excessive daytime sleepiness (EDS) in adults with narcolepsy. The FDA entrectinib (Rozlytrek, Roche) on 8/15/2019 for the treatment of adults with ROS1-positive, metastatic non-small cell lung cancer (NSCLC). Rocheset the monthly WAC at $17,050 for the drug. The FDA approved fedratinib (Inrebic, Celgene, Bristol-Myers Squibb) on 8/16/2019 for the treatment of primary or secondary myelofibrosis classified as either intermediate-2 or high risk. Fedratinib was approved with a Boxed Warning regarding a risk of serious and potentially fatal brain damage or dysfunction. The FDA approved upadacitinib (RINVOQ, AbbVie) on 8/16/2019 for the treatment of adults with moderately to severely active rheumatoid arthritis with an inadequate response or intolerance to methotrexate. AbbVie set the annual WAC at $59,000 for the drug. The EMA accepted the MAA for filgotinib for the treatment of rheumatoid arthritis. Announced Research Updates Verona announced that in a 35 patient, Phase II, open-label trial, treatment with ensifentrine dry powder inhaler increased FEV1 102 mL with the 150-µg dose, 175 mL with the 500-µg dose, 180 mL with the 1,500-µg dose, and 260 mL with the 3000-µg dose in patients with moderate to severe COPD. Deciphera announced that in a 129 patient, Phase III, INVICTUS trial, treatment with ripretinib resulted in a progression-free survival of 6.3 months compared to 1 month with placebo in patients with GIST, whose tumors have progressed after treatment with imatinib, then sunitinib and finally regorafenib. There was a non-significant difference in a secondary endpoint in the INVICTUS trial, where the objective response rate was 9.4% with ripretinib compared to 0% with placebo. Ripretinib will compete with avapritinib for the treatment of GIST, when both drugs are available. Deciphera plans to file an NDA for ripretinib in 1Q20 for the treatment of gastrointestinal stroll tumors. Regeneron announced the in the 499 patient, Phase II/III PALM Trial, mortality was 29% after treatment with REGN-EB3, 34% after mAb114 , 54% with remdesivir compared to 49% with porgaviximab (ZMapp) in patients infected with the Ebola virus. EBOLA UPDATE: The PALM trial compared REGN-EB3, mAb114 and remdesivir to porgaviximab (ZMapp) in patients infected with the Ebola virus. The trial was stopped early, when an interim analysis demonstrated superiority of REGN-EB3 and mAb114 to porgaviximab. Patients in the safety extension phase of the PALM trial will be treated with with REGN-EB3 and mAb114. Once the trial is completed, an expanded access phase owl be initiated. A final analysis of the PALM trial is expected to be completed in 4Q19. Mallinckrodt announced that in the 300 patient, Phase III CONFIM trial, treatment with terlipressin resulted in more patients achieving HRS-1 reversal (renal function improvement, avoidance of dialysis, short-term survival) in patients with HRS-1. Mallinckrodt plans to file an NDA for terlipressin in the first half of 2020 for the treatment of hepatorenal syndrome type 1. Published Research Updates In a 1-month, 26 patient, Phase II trial, treatment with 75 mg of midomafetamine by Multidisciplinary Association for Psychedelic Studies (MAPS) resulted in a 58.3 point reduction in the Clinician-Administered PTSD Scale (CAPS-IV) compared to a 44.3 point reduction with 125 mg and an 11.4 point reduction with 30 mg in military personnel and first responders with post-traumatic stress disorder (PTSD) being treated concurrently with psychotherapy. In a 7-day, 324 patient trial, symptom resolution was achieved in a similar percentage of patients that received 3-days of UTILITY Therapeutics/Leo Pharma’s pivmecillinam compared to 5-days of treatment (76% vs 73%) in women with uncomplicated urinary tract infection. In a 2-year, 611 patient, Phase II, extension, open-label trial, 52% of patients completed the trial and 44% achieved ACR20 in patients with moderate-to-severe rheumatoid arthritis that had participated in Astellas/Janssen’s peficitinib trial. In a 129 patient, Phase IIb, open-label trial, treatment with SillaJen Biotherapeutics/Transgene’s pexastimogene devacirepvec added to best supportive care did not improve the overall survival compared to only best supportive care in patients with hepatocellular carcinoma. In a 12-week, 50 patient, open-label trial, 63.3% of patients treated withHelsinn Birex Pharmaceuticals’ anamorelin had an increase in lean body mass with a mean increase of 1.89 kg in Japanese patients with advanced and unresectable gastrointestinal (colorectal, gastric, or pancreatic) cancer. In the current Ebola outbreak in the Democratic Republic of the Congo (DRC), four drugs were tested as treatment for the viral infection. Two are mixtures of three monoclonal antibodies for the Ebola virus. REGN-EB3 is being developed by Regeneron Pharmaceuticals and porgaviximab (ZMapp) is being developed by Mapp Biopharmaceuticals. A single monoclonal antibody to treat Ebola, mAb114, was discovered by the National Institute of Allergy and Infectious Diseases and licensed to Ridgeback Biotherapeutics for development. The fourth drug is remdesivir, a small molecule anti-viral being developed by Gilead Sciences.
During the 2014-2016 West African Ebola outbreak, ZMapp demonstrated some hope as a potential Ebola treatment. In a 72 patient, randomized, controlled trial with 72 Ebola infected patients, ZMapp demonstrated a 22% mortality rate compared to a 37% mortality rate in the placebo arm. However, the observed posterior probability of 91.2% did not meet the prespecified statistical threshold for efficacy of 97.5%. In the current DRC outbreak, the Phase II/III PALM trial was initiated to compare REGN-EB3, mAb114 and remdesivir with ZMapp. It was planned that 725 patients would be enrolled. The NIH announced that an interim analysis of 499 patient in the PALM Trial, demonstrated that mortality was 29% after treatment with REGN-EB3, 34% after mAb114, 54% with remdesivir compared to 49% with porgaviximab (ZMapp) in patients infected with the Ebola virus. The trial was stopped early due to superiority of REGN-EB3 and mAb114 compared to porgaviximab. Patients in the safety extension phase of the PALM trial will only be treated with REGN-EB3 and mAb114. Once the trial is completed, an expanded access phase will be initiated for both drugs. A final analysis of the PALM trial is expected to be completed in 4Q19. Three vaccines are being developed to prevent the transmission of Ebola. The most advanced is the rVSV-ZEBOV vaccine being developed by Merck and New Genetics. The vaccine was originally discovered by the Canadian Public Health Agency. The 1,500 patient, PREVAIL 1 Phase II trial showed an antibody response that was still effective at one year. At the recommendation of WHO, the rVSV-ZEBOV vaccine is being administered in the DRC using the ring vaccination strategy. This strategy involves vaccination of close and high-risk contacts to known Ebola cases along with people with a high risk of exposure such as health workers, ambulance drivers and people who man the safe burial teams. Johnson & Johnson and Bavarian Nordic are developing the Ad26.ZEBOV vaccine. Ad26.ZEBOV has demonstrated sustained immune responses of 8-12 months in Phase I trials. GSK developed the cAd3-EBOZ vaccine in collaboration with the NIH. GSK licensed the vaccine to the Sabin Vaccine Institute. Sabin has signed an agreement with the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center to develop the drug. A recent article in STAT news discussed that developing effective vaccines and treatments are just part of the process to control Ebola outbreaks. Problems remain with instability and violence in the areas preventing medical teams from accessing them, changes in burial customs to prevent contact with infected bodies and isolating infected patients. Click here to subscribe to Prescribe Right’s Pharmaceutical Pipeline Tracker to monitor current developments in the fight to control EBOLA. Regulatory Update
The FDA accepted the NDA for givosiran for the treatment of acute hepatic porphyria and set a PDFUA date of 2/4/2020. The FDA accepted the NDA for avapritinib for the treatment of PDGFRA Exon 18 mutant gastrointestinal stromal tumors and set a PDFUA date of 2/14/2020. After canceling an advisory committee meeting to review lumateperone due to new information provided for the NDA, the FDA delayed the PDUFA date for 3 months to 12/27/2019. The FDAdesignated teplizumaba Breakthrough Therapy Designation for the prevention or delay of clinical type 1 diabetes in patient at risk of developing the disease. GSK licensed its Ebola vaccines to the Sabin Vaccine Institute. Sabin has signed an agreement with the National Institute of Allergy and Infectious Diseases’s (NIAID) Vaccine Research Center to develop the drug. The EMA has accepted the MAA for abiciparfor the treatment of neovascular age-related macular degeneration. DBV withdrew itsoriginal BLA for the Viaskin peanut allergy transdermal patch in 2018 due to FDA concerns about its manufacturing process. DBV resubmitted a BLA in August 2019. Announced Research Updates Rhythm announced that in a 12-month, 11 patient, Phase III, open-label trial, treatment with setmelanotide resulted in a 10% reduction in body weight with a mean body weight reduction of 25.4% in eight patients with pro-opiomelanocortin deficiency obesity. Rhythm announced that in a 12-month, 10 patient, Phase III, open-label trial, treatment with setmelanotide resulted in a 10% reduction in body weight with a mean body weight reduction of 25.4 in five patients with leptin receptor deficiency obesity. Patients in both trials went through a withdrawal period, where weight was gained back before starting back on the drug for an extension trial. Patients lost weight after restarting the drug. Rhythm Pharmaceuticals plans to complete a rolling NDA submission for setmelanotide for the treatment of both pro-opiomelanocortin and leptin receptor deficiency obesity in 4Q19. Rhythm has ongoing extension trials for both pro-opiomelanocortin and leptin receptor deficiency obesities. Rhythm also continues to enroll portents for both deficiencies and is targeting pediatric patients. Basileaannounced that in the 679 patient, Phase III TARGET trial, 91.3% of patients treated with ceftobiprole achieved a 20% or more reduction from baseline in lesion size at 48 to 72 hours compared to 88.1% of patients treated with vancomycin plus aztreonam in patients with acute bacterial skin and skin structure infection. Published Research Updates In a 92 patient, Phase II trial, treatment with cediranib improved progression-free survival compared to placebo (7.2 months v 5.6 months) in patients with unresectable malignant pleural mesothelioma. In the 66 patient, 3-month, Phase III APPROACH trial, volanesorsen decreased triglycerides 77% vs an 18% increase with placebo in patients with familial chylomicronemia syndrome. Arena announced that in a 22-week, 61 patient, Phase II trial, ralinepag decreased pulmonary vascular resistance by 163.9 dyn.sec/cm5 compared to an increase of 0.7 dyn.sec/cm5 with placebo in patients with pulmonary arterial hypertension (PAH). In a 4.9 month, 334 patient, Phase II trial, 50% of patients treated with taselisib added to letrozole achieved an objective response compared to 39% with letrozole monotherapy in postmenopausal women with ER+, HER2-operable breast cancer. However, there was no difference in pathological complete response between groups. During the first half of 2019 FDA approved thirteen new investigational drugs for market distribution. The FDA took action on fourteen investigational drugs in July. There were three new approvals in line with the approval pace of the first half. Two drugs received new Priority Designations from the FDA and the agency accepted four new NDAs and three new BLAs. On the other hand, there were setbacks for Intra-Cellular Therapies and Nektar.
July 2019 Approvals
Regulatory Update
The FDA approved darolutamide (Nubeqa, Bayer) on 7/31/2019 for the treatment of nonmetastatic castration-resistant prostate cancer. Darolutamide was approved three months before its PDUFA date. The FDA approved pexidartinib (Turalio, Daiichi Sankyo) for the treatment of symptomatic tenosynovial giant cell tumor associated with severe morbidity or functional limitations and not responsive to improvement with surgery. Pexidartinib has Boxed Warning for the risk of serious and potentially fatal liver injury. The drugs is only available though a REMS program. On 8/1/2019 Horizon Therapeutics initiated an FDA approved expanded access program for teprotumumab for up to 60 patients with active moderate to severe thyroid eye disease under ClinicalTrials.gov NCT number: NCT04040894. Physicians can request access to teprotumumab sending an e-mail to [email protected]. Horizon Therapeutics submitted a BLA for teprotumumab in July 2019. The FDA accepted the NDA for dasotraline in July 2019 for the treatment of patients with moderate-to-severe binge eating disorder and set a PDUFA date in May 2020. Ultragenyx has submitted an NDA for the use of triheptanoin in the treatment of long-chain fatty acid oxidation disorders. The FDA designated elafibranor an orphan drug for the treatment of primary biliary cholangitis. The FDA designated bempegaldesleukin in combination with nivolumab as a Breakthrough Therapy for initial treatment of unresectable or metastatic melanoma. Announced Research Updates Cidara announced that in 91 patients were enrolled in Part B of the STRIVE trial. Halfway through Part B of the trial, the dosing for rezafungin was changed to the dosing used in the Phase III trials, so only 15 rezafungin patients out of 46 received the higher dose. Treatment with rezafungin resulted in a higher clinical cure rate than caspofungin, but the small sample size and change in dosing limited the value and significance of the findings. Sanofi announced that in the 26-week, Phase III SOTA-MET trial, treatment with sotagliflozin reduced HbA1c compared to placebo in type 2 diabetic patients receiving metformin. Sanofi announced that in the 26-week, Phase III SOTA-CKD3T trial, treatment with sotagliflozin reduced HbA1c compared to placebo in all patients with type 2 diabetes chronic kidney disease, but in a subgroup glomerular filtration rate of 30-<45 mL/min/1.73m2, there was no improvement with sotagliflozin over placebo. Sanofi announced that in the 26-week, Phase III SOTA-CKD4 trial, treatment with sotagliflozin did not improve HbA1c compared to placebo in type 2 diabetic patients with stage 4 chronic kidney disease. Sanofi ceased participation in the development of sotagliflozin after results from the 3 Phase III type 2 diabetes trials were announced. Biogen announced that in the 5-week, 506 patient, Phase III, EVOLVE-MS-2 trial, where patients treated with diroximel fumarate reported fewer days with GI symptoms with a symptom intensity score ≥2 in patients with relapsing-remitting multiple sclerosis. AB Science discontinued development of masitinib for melanoma after difficulties completing a Phase III trial, where the dacarbazine comparator arm was halted for ethical reasons at the request of regulatory agencies. Development of masitinib continues for the treatment of pancreatic cancer and prostate cancer. Pfizer announced that in the 42-day, 345 patient, Phase III, RESET trial, treatment with rivipansel did not improve time to readiness-for-discharge compared to placebo in patients with sickle cell disease who were hospitalized for a vaso-occlusive crisis and required treatment with intravenous (IV) opioids Published Research Updates In a 12-week, 52 patient open label trial, switching patients to blonanserin decreased the Brief Psychiatric Rating Scale score by 13.7 points in schizophrenic patients unresponsive to other antipsychotic treatments. In a 12-week, 52 patient open label trial, switching patients to blonanserin decreased the Brief Psychiatric Rating Scale score by 13.7 points in schizophrenic patients unresponsive to other antipsychotic treatments. In a 49 patient, Phase Ia/Ib, dose ranging trial, 20% of patients treated with the combination of tislelizumab and pamiparib achieved an objective response in patients with advanced solid tumors. There are seven investigational drugs with PDUFA Dates in August 2019.
|
Stay informed, subscribe to the
Prescribe Right Pharmaceutical Pipeline Tracker Latest Tweets from Prescribe Right
Archives
July 2023
|
Services |
Company |
Support |
© COPYRIGHT 2015. ALL RIGHTS RESERVED.
|


 RSS Feed
RSS Feed